Potassium Carbonate
Price on request
Buy Potassium Carbonate
online from Chemical Solution Inc. (CSI): Your trusted distributor of high quality chemical raw materials and laboratory equipment.
| Chemical Name | Potassium Carbonate |
| Synonyms | Carbonic Acid Dipotassium Salt; Potassium Carbonate (K2CO3); CLIMB; DCAD Plus; Dipotassium Carbonate; FG; Pearl Ash; Potash; Potassium Carbonate; Potassium Carbonate (2:1); Potassium Carbonate (K2(CO3)); Salt of Tartar; Sorb KX 35 |
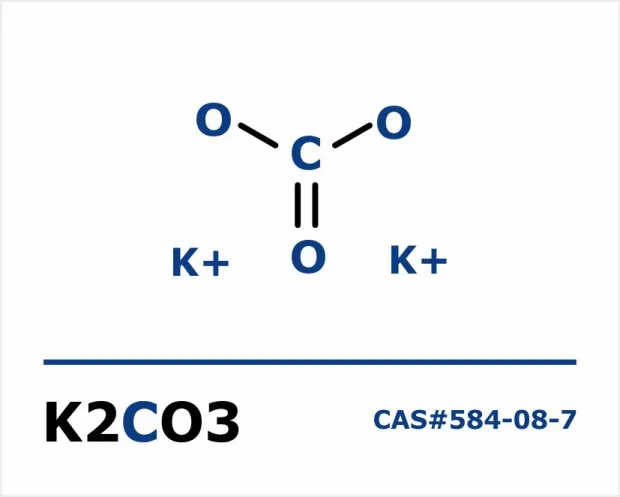
| CAS Number | 584-08-7 |
| Alternate CAS # | Free acid: 463-79-6;Deleted CAS: 30095-94-4, 16437 |
| Molecular Formula | CK₂O₃ |
| Appearance | White to Off-White Solid |
| Melting Point | >300°C |
| Molecular Weight | 138.21 |
| Storage | 20°C |
| Solubility | Water |
| Category | Building Blocks; Inorganics; |
| Applications | Potassium Carbonate is a common chemical reagent used in various organic reactions. It is used, for example, in the synthesis of Diazoxon (D416890); the oxidized form of Diazinon (D416880) which is an organophosphate insecticide. Diazoxon is shown to be a more potent acetylcholinesterase (AChE) inhibitor. |
Potassium Carbonate: Properties, Uses, and Benefits
Potassium carbonate, also known as pearl ash, is an inorganic salt with the chemical formula K2CO3. It is a white, odorless, and water-soluble compound that has been used in various industrial, agricultural, and medicinal applications for centuries. In this article, we will explore the properties, uses, and benefits of potassium carbonate in detail.
Chemical Properties of Potassium Carbonate
Potassium carbonate is an inorganic salt that is made up of two potassium cations (K+) and one carbonate anion (CO3^2-). It has a molar mass of 138.205 g/mol and a melting point of 891°C. Potassium carbonate is a basic salt and reacts with acids to produce potassium salts and carbon dioxide gas.
Physical Properties of Potassium Carbonate
Potassium carbonate is a white, crystalline powder that is odorless and has a salty taste. It is soluble in water and glycerol but insoluble in ethanol and ether. Potassium carbonate has a density of 2.43 g/cm3 and a specific gravity of 2.43. It is hygroscopic, meaning that it can absorb water from the air.
Synthesis of Potassium Carbonate
Potassium carbonate can be synthesized through several methods, including the Leblanc process, Solvay process, and electrolysis of potassium chloride. The Leblanc process involves reacting sodium chloride with sulfuric acid to produce sodium sulfate and hydrogen chloride gas. The hydrogen chloride gas is then reacted with limestone to produce calcium chloride and carbon dioxide gas. The calcium chloride is then reacted with potassium carbonate to produce calcium carbonate and potassium chloride. The Solvay process involves reacting sodium chloride with ammonia and carbon dioxide gas to produce sodium bicarbonate and ammonium chloride. The sodium bicarbonate is then reacted with calcium hydroxide to produce calcium carbonate and carbon dioxide gas. The calcium carbonate is then reacted with potassium chloride to produce potassium carbonate and calcium chloride.
Uses of Potassium Carbonate in Industry
Potassium carbonate is used in various industries, including glass, ceramics, soap, and fertilizer production. In the glass industry, potassium carbonate is used as a fluxing agent to reduce the melting point of silica and other materials. In the ceramics industry, it is used as a glaze component to improve the durability and color of the ceramic. In the soap industry, it is used as a softening agent to make the soap more soluble and stable. In the fertilizer industry, it is used as a source of potassium to improve plant growth and yield.
Uses of Potassium Carbonate in Agriculture
Potassium carbonate is a common ingredient in fertilizers and soil amendments. It is used as a source of potassium to improve soil fertility and plant growth. Potassium carbonate can also be used to adjust the pH of the soil, making it more alkaline. It is particularly useful in alkaline soils that have a low availability of potassium.
Uses of Potassium Carbonate in Medicine
Potassium carbonate has several medicinal uses, including as an antacid and as a source of potassium supplementation. As an antacid, it can be used to neutralize excess stomach acid and relieve symptoms of heartburn and indigestion. As a source of potassium, it can be used to treat hyp
okalemia, a condition where the body has low levels of potassium. Potassium carbonate is also used in the production of some pharmaceuticals, such as potassium bicarbonate tablets and solutions.
Benefits of Potassium Carbonate
Potassium carbonate offers several benefits in various applications. As a source of potassium, it is essential for proper nerve and muscle function, fluid balance, and heart health. It also helps regulate blood pressure and supports bone health. In the glass and ceramics industry, potassium carbonate helps improve product quality and reduces production costs. In the soap industry, it helps improve soap solubility and stability, leading to a longer shelf life. In agriculture, it helps improve crop yield and quality, leading to higher profits for farmers.
Safety and Precautions
Potassium carbonate is generally safe to handle and use. However, it can cause skin and eye irritation if it comes into contact with these areas. Ingesting large amounts of potassium carbonate can also be harmful and may cause gastrointestinal discomfort, vomiting, and diarrhea. Inhaling potassium carbonate dust can also cause respiratory irritation. Therefore, it is important to wear protective equipment, such as gloves, goggles, and a mask, when handling potassium carbonate.
Conclusion
Potassium carbonate is an important inorganic salt that offers various benefits in industry, agriculture, and medicine. Its properties and uses make it a versatile compound that is essential in many applications. However, it is important to handle and use it with care and take necessary precautions to avoid potential harm.